|
 |

For informational purposes: COVID-19 therapeutic options
Established and potential repurposed therapeutics for SARS-CoV-2 infection (COVID-19) [PDF].
This one-page listing does not comprise a set of recommendations, but includes generally well-tolerated medications with potential empiric benefit in COVID-19. These candidates include medications approved by the FDA for other conditions, or represent over-the-counter supplements. While their mode of action is consistent with suspected cellular and molecular disease mechanisms, and beneficial outcomes have been indicated in research studies and clinical reports with few adverse side-effects, their use in COVID-19 has generally not been evaluated in large-scale randomized controlled trials. Typical contraindications apply. These references are provided strictly to share and discuss with a physician, and are not intended as medical advice.
Severe clinical worsening in COVID-19 and potential mechanisms of immune-enhanced disease
[Full Text]
John P. Hussman, Ph.D., Frontiers in Medicine, June 2021
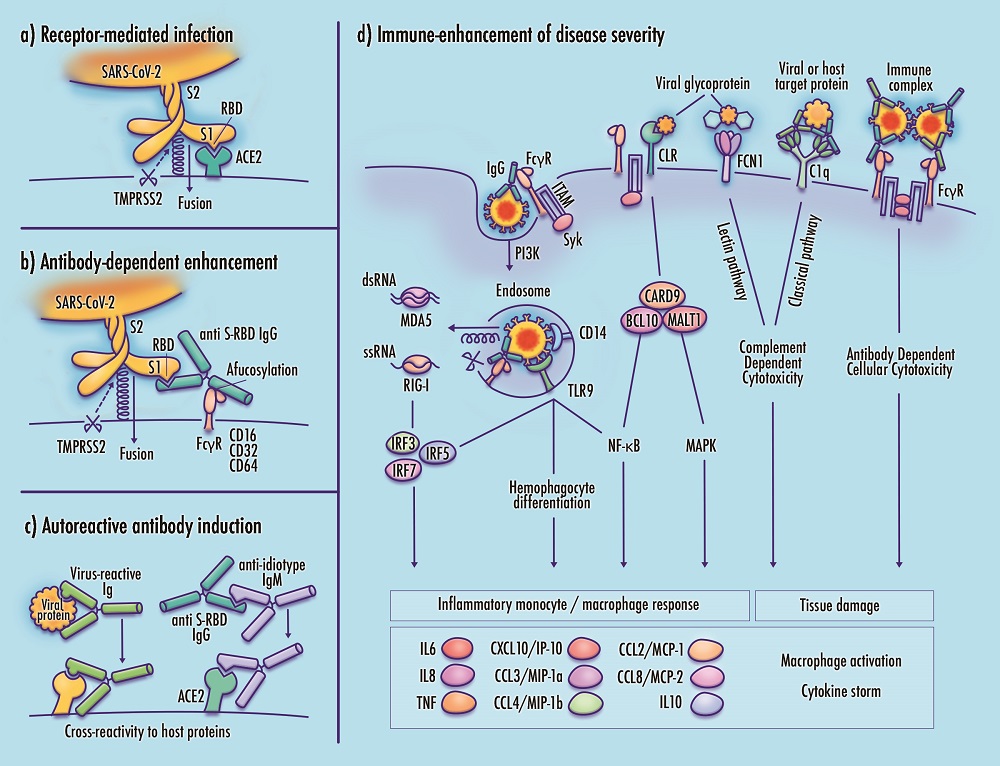
ABSTRACT
Infection by the novel SARS-CoV-2 coronavirus produces a range of outcomes, with the majority of cases producing mild or asymptomatic effects, and a smaller subset progressing to critical or fatal COVID-19 disease featuring severe acute respiratory distress. Although the mechanisms driving severe disease progression remain unknown, it is possible that the abrupt clinical deterioration observed in patients with critical disease corresponds to a discrete underlying expansion of viral tropism, from infection of cells comprising respiratory linings and alveolar epithelia to direct infection and activation of inflammatory monocytes and macrophages. Dysregulated immune responses could then contribute to disease severity. This article discusses the potential role of monocyte/macrophage (Mo/Mφ) infection by SARS-CoV-2 in mediating the immune response in severe COVID-19. Additional mechanisms of immune-enhanced disease, comprising maladaptive immune responses that may aggravate rather than alleviate severity, are also discussed. Severe acute clinical worsening in COVID-19 patients may be influenced by the emergence of antibodies that participate in hyperinflammatory monocyte response, release of neutrophil extracellular traps (NETs), thrombosis, platelet apoptosis, viral entry into Fc gamma receptor (Fcγ)-expressing immune cells, and induction of autoantibodies with cross-reactivity against host proteins. While the potential roles of Mo/Mφ infection and immune-enhanced pathology in COVID-19 are consistent with a broad range of clinical and laboratory findings, their prominence remains tentative pending further validation. In the interim, these proposed mechanisms present immediate avenues of inquiry that may help to evaluate the safety of candidate vaccines and antibody-based therapeutics, and to support consideration of pathway-informed, well-tolerated therapeutic candidates targeting the dysregulated immune response.
Cellular and Molecular Pathways of COVID-19 and potential points of therapeutic intervention
[Full Text]
John P. Hussman, Ph.D., Frontiers in Pharmacology, July 2020
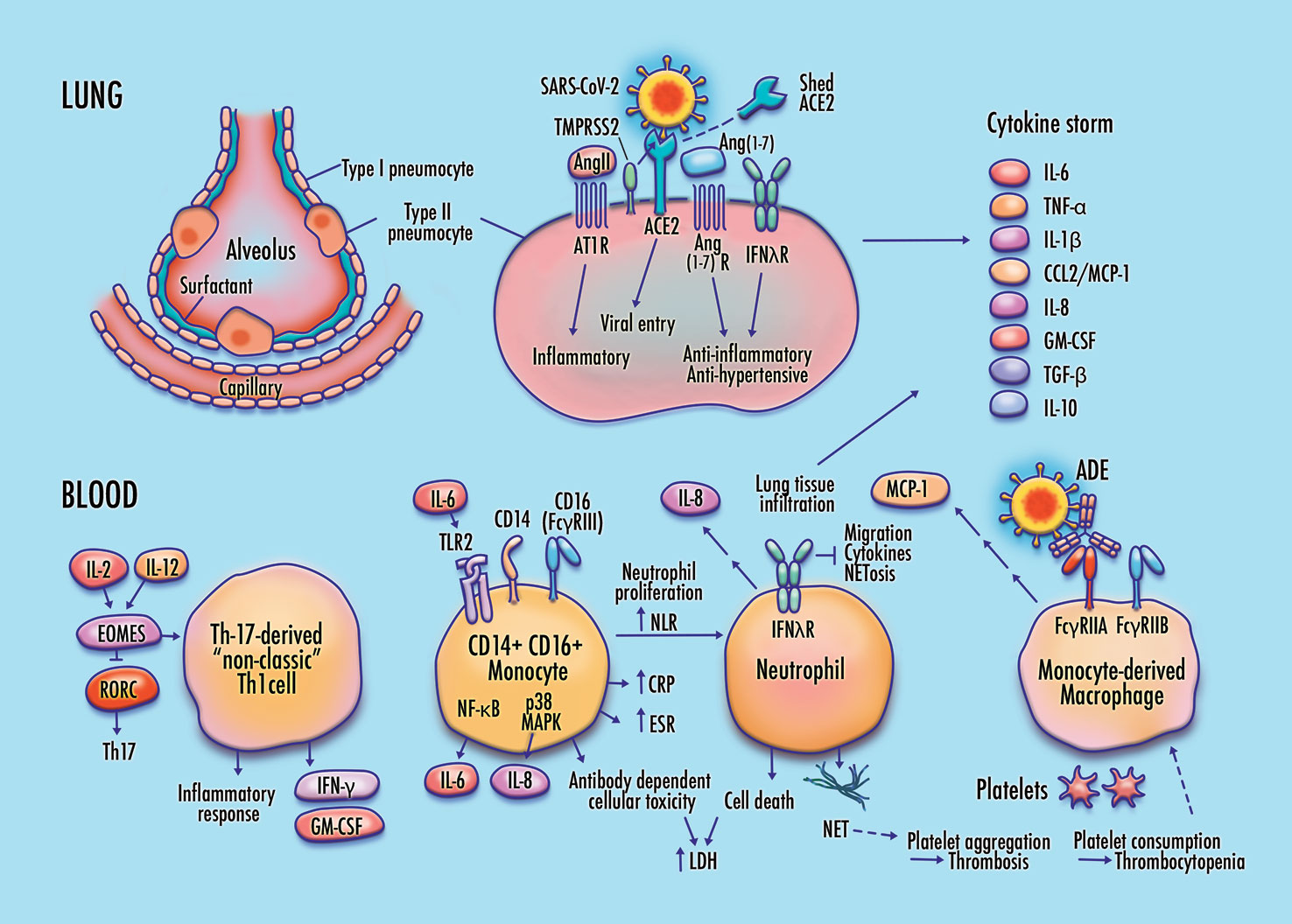
ABSTRACT
Proposed features of cellular and molecular pathophysiology in COVID-19. Membrane fusion and cytoplasmic entry of SARS-CoV-2 virus via ACE2 and TMPRSS2-expressing respiratory epithelial cells, including pulmonary type-II pneumocytes, provokes an initial immune response featuring inflammatory cytokine production coupled with a weak interferon response, particularly in IFN-λ dependent epithelial defense. Differentiation of non-classic pathogenic T-cells and pro-inflammatory intermediate monocytes contributes to a skewed inflammatory profile, mediated by membrane-bound immune receptor subtypes (e.g., FcγRIIA) and downstream signaling pathways (e.g., NF-κB p65 and p38 MAPK), followed by chemotactic infiltration of monocyte-derived macrophages and neutrophils into lung tissue. Endothelial barrier degradation and capillary leakage contribute to alveolar cell damage. Inflammatory cytokine release, delayed neutrophil apoptosis, and NETosis contribute to pulmonary thrombosis and cytokine storm. These mechanisms are concordant with observed clinical markers in COVID-19, including high expression of inflammatory cytokines on the TNF-α/IL-6 axis, elevated neutrophil-to-lymphocyte ratio (NLR), DAD via cell apoptosis in respiratory epithelia and vascular endothelia, elevated lactate dehydrogenase (LDH), erythrocyte sedimentation rate (ESR), and CRP, high production of neutrophil extracellular traps (NETs), depressed platelet count, and thrombosis.
GWAS analysis implicates NF-κB-mediated induction of inflammatory T cells in multiple sclerosis
[Full Text]
John P. Hussman, Ashley H Beecham, Michael M. Schmidt, Eden R. Martin, Jacob J. McCauley, Jeffrey M. Vance, Jonathan L. Haines, Margaret A. Pericak-Vance, Nature - Genes & Immunity, June 2016
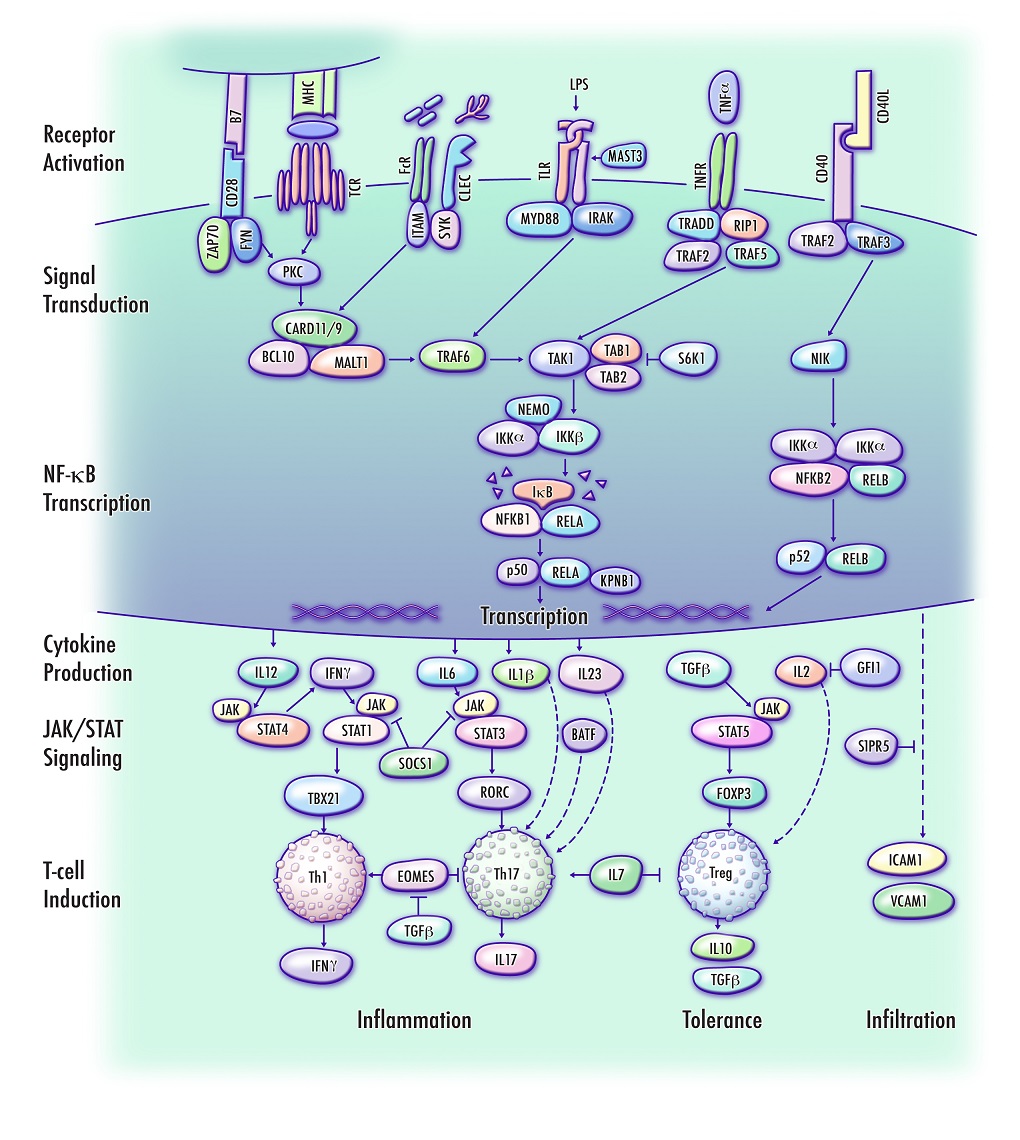
ABSTRACT
To identify genes and biologically relevant pathways associated with risk to develop multiple sclerosis (MS), the Genome-Wide Association Studies noise reduction method (GWAS-NR) was applied to MS genotyping data. Regions of association were defined based on the significance of linkage disequilibrium blocks. Candidate genes were cross-referenced based on a review of current literature, with attention to molecular function and directly interacting proteins. Supplementary annotations and pathway enrichment scores were generated using The Database for Annotation, Visualization and Integrated Discovery. The candidate set of 220 MS susceptibility genes prioritized by GWAS-NR was highly enriched with genes involved in biological pathways related to positive regulation of cell, lymphocyte and leukocyte activation (P=6.1E-15, 1.2E-14 and 5.0E-14, respectively). Novel gene candidates include key regulators of NF-κB signaling and CD4+ T helper type 1 (Th1) and T helper type 17 (Th17) lineages. A large subset of MS candidate genes prioritized by GWAS-NR were found to interact in a tractable pathway regulating the NF-κB-mediated induction and infiltration of pro-inflammatory Th1/Th17 T-cell lineages, and maintenance of immune tolerance by T-regulatory cells. This mechanism provides a biological context that potentially links clinical observations in MS to the underlying genetic landscape that may confer susceptibility.
Autism and disruption of excitatory/inhibitory balance
In 2001, Dr. John P. Hussman first proposed that suppressed GABAergic inhibition, with resulting glutamate imbalance and disrupted excitatory-inhibitory regulation, could be a central factor in autism. Based on convergent lines of evidence from genetic, neuroanatomical, and neurobiological research, Hussman proposed:
"Although it is possible that the breadth of impairments in autism are caused by multiple defects in relatively independent systems, autism may instead reflect dysfunction in a single factor shared in common by many systems. Specifically, the severe disruptions observed in autism may be linked to suppression of GABAergic inhibition, resulting in excessive stimulation of glutamate-specialized neurons and loss of sensory gating. This view recognizes the possibility of multiple etiologies in autism. That is, there may exist a spectrum of genetic and environmental factors which impair inhibitory tone in a manner that expresses itself as autistic pathology."
"Such a model may provide a reasonable characterization of autism. That is, loss of inhibitory control may cause a deterioration in the quality of sensory information due to the failure to suppress competing 'noise.' The ability to process sensory information and learning tasks would then be overwhelmed. Indeed, many autistic behaviors appear to be mechanisms for restricting sensory input to a narrow, repetitive or controllable scope."
"In summary, the hypothesis of impaired amino acid neurotransmission in autism is consistent with a broad range of findings from neuroanatomic and neurobiological research. Moreover, several functional deficits in autism are consistent with dysfunction in brain regions dependent on GABAergic inhibitory tone, or having specialized responses or selective vulnerability to glutamate. The limbic structures, frontal cortex, and cerebellum appear particularly important due to their probable role in the integration of sensory input. Among several suspected etiologies of autism, the possibility of impaired inhibitory tone emerges as a common factor."
In the years that have followed, the mechanism originally proposed by Dr. Hussman has become one of the most well-established lines of research in the field. We are encouraged that the work of the Hussman Foundation continues to advance the goal of improving the lives of individuals with autism.
Suppressed GABAergic inhibition as a common factor in suspected etiologies of autism
[Full Text PDF]
John P. Hussman, Ph.D., Journal of Autism & Developmental Disorders, April 2001
As a postscript, Hussman's (2001) research was later cited by Rubenstein & Merzenich (2003), who described reduced inhibition and excess excitatory drive in terms of "an increased ratio of excitation/inhibition" (E/I). Because their citation did not clarify that the model was substantially that of Dr. Hussman, the hypothesis of altered excitatory/inhibitory neurotransmission in autism is often inadvertently misattributed to Rubenstein & Merzenich.
Notably, a preponderance of subsequent research has identified that the primary disruption of E/I balance in autism is specific to GABA/glutamate neurotransmission. While the concept of "noise" (which Hussman originally drew from his dissertation work on informational noise-reduction) was adopted by Rubenstein & Merzenich as "cortical noise," it is important to recognize that the processing disruptions identified in autism are not specific to cortical processes. Indeed, limbic structures such as the basal ganglia, as well as cerebellar processing, remain of central interest in autism.
Much of the Foundation's current research efforts focuse on altered connectivity and synchrony in autism, particularly involving a subset of GABAergic interneurons that express parvalbumin (PV), a calcium-buffering protein that makes these interneurons important in coordinating signaling across multiple processing areas in the brain. This book chapter by Dr. Hussman describes that mechanism, and why it implies a presumption of competence toward individuals with autism.
The gap between intention and action: altered connectivity and GABA-mediated synchrony in autism
[Full Text PDF]
John P. Hussman, Ph.D.
Published in: Autism: The Movement Sensing Perspective, 2017, Torres, E.B. & Whyatt C., Editors, CRC Press
|
News Release: Mapping the Genetic Pathway for Autism

A noise-reduction GWAS analysis implicates altered regulation of neurite outgrowth and guidance in autism
[Full Text PDF]
John P Hussman , Ren-Hua Chung , Anthony J Griswold , James M Jaworkski , Daria Salyakina , Deqiong Ma , Ioanna Konidari , Patrice L Whitehead , Jeffery M Vance , Eden R Martin , Michael L Cuccaro , John R Gilbert , Jonathan L Haines and Margaret A Pericak-Vance
[Full Text HTML] Molecular Autism 2011, 2 :1 doi:10.1186/2040-2392-2-1
[Additional Files including candidate genes and supporting literature]
Published: 19 January 2011
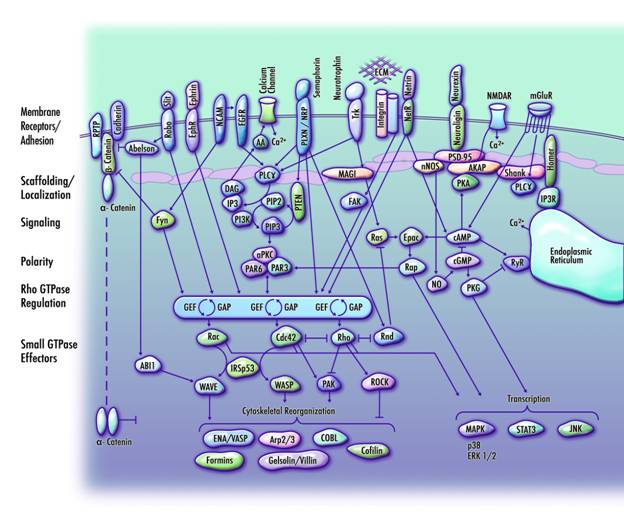
ABSTRACT
Background
Genome-wide Association Studies (GWAS) have proved invaluable for the identification of disease susceptibility genes. However, the prioritization of candidate genes and regions for follow-up studies often proves difficult due to false-positive associations caused by statistical noise and multiple testing. In order to address this issue, we propose the novel GWAS noise reduction (GWAS-NR) method as a way to increase the power to detect true associations in GWAS, particularly in complex diseases such as autism.
Methods
GWAS-NR utilizes a linear filter to identify genomic regions demonstrating correlation among association signals in multiple datasets. We used computer simulations to assess the ability of GWAS-NR to detect association against the commonly used joint analysis and Fisher's methods. Furthermore, we applied GWAS-NR to a family-based autism GWAS of 597 families and a second existing autism GWAS of 696 families from the Autism Genetic Resource Exchange (AGRE) to arrive at a compendium of autism candidate genes. These genes were manually annotated and classified by a literature review and functional grouping in order to reveal biological pathways which might contribute to autism aetiology.
Results
Computer simulations indicate that GWAS-NR achieves a significantly higher classification rate for true positive association signals than either the joint analysis or Fisher's methods and that it can also achieve this when there is imperfect marker overlap across datasets or when the closest disease-related polymorphism is not directly typed. In two autism datasets, GWAS-NR analysis resulted in 1535 significant linkage disequilibrium (LD) blocks overlapping 431 unique reference sequencing (RefSeq) genes. Moreover, we identified the nearest RefSeq gene to the non-gene overlapping LD blocks, producing a final candidate set of 860 genes. Functional categorization of these implicated genes indicates that a significant proportion of them cooperate in a coherent pathway that regulates the directional protrusion of axons and dendrites to their appropriate synaptic targets.
Conclusions
As statistical noise is likely to particularly affect studies of complex disorders, where genetic heterogeneity or interaction between genes may confound the ability to detect association, GWAS-NR offers a powerful method for prioritizing regions for follow-up studies. Applying this method to autism datasets, GWAS-NR analysis indicates that a large subset of genes involved in the outgrowth and guidance of axons and dendrites is implicated in the aetiology of autism.
----
The following papers are part of an ongoing research partnership with Dr. Margaret Pericak-Vance and Dr. Jeffery Vance, now of the Hussman Institute of Human Genomics at the University of Miami, to identify genetic factors involved in autism and other complex conditions:
GWAS analysis implicates NF-κB-mediated induction of inflammatory T cells in multiple sclerosis.
Hussman JP, Beecham AH, Schmidt M, Martin ER, McCauley JL, Vance JM, Haines JL & Pericak-Vance MA, Nature, Genes & Immunity, 17, 305-312 (2016).
A genome-wide association study of autism reveals a common novel risk locus at 5p14.1.
Ma D, Salyakina D, Jaworski JM, Konidari I, Whitehead PL, Andersen AN, Hoffman JD, Slifer SH, Hedges DJ, Cukier HN, Griswold AJ, McCauley JL, Beecham GW, Wright HH, Abramson RK, Martin ER, Hussman JP, Gilbert JR, Cuccaro ML, Haines JL, Pericak-Vance MA. Ann Hum Genet . 2009 May;73(Pt 3):263-73.
Identification of significant association and gene-gene interaction of GABA receptor subunit genes in autism.
Ma DQ, Whitehead PL, Menold MM, Martin ER, Ashley-Koch AE, Mei H, Ritchie MD, Delong GR, Abramson RK, Wright HH, Cuccaro ML, Hussman JP, Gilbert JR, Pericak-Vance MA. American Journal of Human Genetics. 2005 Sep;77(3):377-88
An analysis paradigm for investigating multi-locus effects in complex disease: examination of three GABA receptor subunit genes on 15q11-q13 as risk factors for autistic disorder.
Ashley-Koch AE, Mei H, Jaworski J, Ma DQ, Ritchie MD, Menold MM, Delong GR, Abramson RK, Wright HH, Hussman JP, Cuccaro ML, Gilbert JR, Martin ER, Pericak-Vance MA. Ann Hum Genet. 2006 May;70(Pt 3):281-92.
Investigation of autism and GABA receptor subunit genes in multiple ethnic groups.
Collins AL, Ma DQ, Whitehead PL, Martin ER, Wright HH, Abramson RK, Hussman JP, Haines JL, Cuccaro ML, Gilbert JR, Pericak-Vance MA. Neurogenetics. 2006 Jul;7(3):167-74. Epub 2006 Jun 13
Other published studies and research supported by the Hussman Foundation:
Decreased GAD67 mRNA levels in cerebellar Purkinje cells in autism: pathophysiological implications.
Yip J, Soghomonian JJ, Blatt GJ. Acta Neuropathol (Berl). 2007 Jan 18; Epub.
|
|

|
|




